Fetal And Neonatal Monitoring Devices Can Help Diagnose Certain Disorders And Illnesses That Can Affect The Fetus
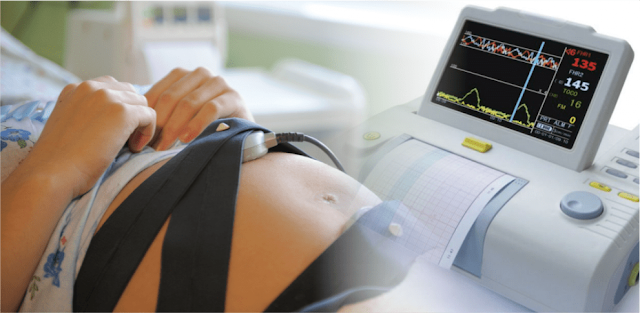
There are many types of fetal and neonatal monitoring devices available. These devices can be used during pregnancy, labor, and delivery to measure the baby's heart rate and other vital signs. Various factors can influence the outcome of these tests, including the risks of trauma and tissue damage.
In
addition to being vital for the fetus's development, neonatal monitoring
devices can help diagnose certain disorders and illnesses that can affect the
fetus. These devices monitor the heart rate, oxygen saturation, and
contractions of the mother's uterus to detect fetal anomalies. Portable Doppler
instruments, electronic fetal monitors, and continuous neonatal monitoring
devices can be used anywhere.
Technological
developments and stringent regulatory policies have fueled the global market
for Fetal
And Neonatal Monitoring Devices. Manufacturers of these devices must undergo several tests and meet various requirements laid down by
different regulatory bodies. The US Food and Drug Administration, the NMPA, and
the European Union's GDPR regulations are some of the bodies that enforce these
requirements. Increasing product approvals are expected to drive the market
during the forecast period.
Increasing
adoption of neonatal monitoring devices is one of the key growth drivers for
the fetal monitoring industry. This technology helps health professionals
monitor the growth of newborns and prevent potential problems. Developed to
help parents with a high-risk pregnancy, Neonatal Monitoring Devices monitor
the health of both the mother and the baby. With the help of these devices,
healthcare providers can monitor the progress of a pregnancy, identify risks
and improve care quality.
Neonatal
monitoring devices are a necessary part of gynecology and obstetrics. They
monitor fetal health during labor and are indispensable for modern
prenatal care. Growth in the market is primarily due to the high number of
preterm births, which cause an estimated 1 million deaths every year. Premature
babies are also more vulnerable to infection and diseases than full-term
newborns. To prevent these devastating outcomes, neonatal monitoring devices
help health care providers monitor the health of premature babies and keep them
under strict surveillance.
The
technology behind neonatal monitoring devices is a complex machine that
continuously measures and displays vital physiological parameters. The devices
are used to monitor a baby's heart rate, respiration rate, blood pressure, and
peripheral capillary oxygen saturation. They are designed to monitor a
newborn's heart rate and respiration rate, and can also record the temperature.
These devices can help monitor a newborn's body temperature and help prevent
serious complications.
The
regulatory policies governing the neonatal monitoring devices market are one of
the major factors that limit their market growth. Manufacturers of neonatal
monitoring devices must undergo several tests and meet stringent requirements
set by various regulatory bodies. The FDA's Center for Devices and Radiological
Health, NMPA regulations, and EU directives are some of the main regulatory
bodies regulating this market. Moreover, the changes related to data protection
for customers will increase the overall cost of healthcare. The key players in
the market are Becton, Dickinson and Company, Fujifilm SonoSite Inc., Medtronic
Plc., Cooper Surgical, Inc., Getinge AB, Natus Medical Incorporated, and
Siemens Healthcare GMBH



Comments
Post a Comment